Generative Chemistry: Navigating Vast Chemical Space in Drug Discovery
Explore the tremendous potential that generative chemistry offers drug discovery.

Complete the form below to unlock access to ALL audio articles.
We’re at an interesting crossroads in drug discovery. Despite our growing understanding of disease biology, finding the right molecule to effectively target and intervene in disease pathways remains incredibly challenging.
At the heart of this challenge is the sheer magnitude of chemical space. There are approximately 1060 small molecules that are typically considered “drug-like”.1 To give some scale to this number, it exceeds the total atoms in our entire solar system by several thousand times. Moreover, as we increasingly explore beyond these traditional parameters, with larger molecules like macrocycles and peptides proving effective for some more challenging targets, the true vastness of potential therapeutic chemistry becomes almost incomprehensible.
No team of scientists, regardless of their expertise or resources, could explore even a meaningful fraction of such a space through traditional methods alone. This is where generative chemistry offers tremendous potential.
An overview of generative chemistry
Generative chemistry harnesses machine learning algorithms to systematically identify new chemical structures based on defined parameters. By learning from chemical databases and scientific literature, generative chemistry can predict and propose new molecules with specific desired properties.
It's worth noting that this field predates the AI boom, which is a testament to its value beyond hype. Traditional generative methods have long combined known scaffolds, fragments and reagents to efficiently explore relevant chemical space.
Now, AI-powered generative chemistry has changed the sophistication and scale of these approaches. This employs advanced neural networks that fall broadly into two categories:
- Graph-based models represent molecular structures as geometric graphs in which the nodes correspond to atoms and edges to bonds. For example, diffusion models use iterative de-noising techniques (similarly to image generators) that gradually convert random noise into coherent molecular structures. They are particularly valuable in their ability to work in 3D,2 although this complexity makes them slower to run.
- Sequence models treat chemical representations as text-like sequences, applying language model techniques to generate new compounds. These models are better established and more efficient to run, although they only work in two dimensions.
Both approaches offer their own advantages, though the distinction ultimately matters less than how these models are deployed within the drug discovery workflow.
Humans and generative chemistry working together
Despite its potential, the most effective approach to generative chemistry isn't about replacing human expertise. Instead, it's enhancing our knowledge through what we call "Augmented Chemistry", which leverages the complementary strengths of both:
- Chemists bring contextual understanding of the project and its objectives and can provide much faster insights on the synthetic feasibility of new compounds.
- AI algorithms can systematically and comprehensively explore vast chemical space without the biases or limitations inherent to humans.
In practice, medicinal chemists can guide machine learning models toward promising regions of chemical space, while algorithms generate and prioritize diverse molecules that might challenge conventional thinking. The result is an iterative cycle where humans and technology learn from and reinforce one another.
Using AI alone risks producing a long list of compounds that look promising on paper but include structures that are synthetically inaccessible, unstable or otherwise problematic. By incorporating critical scientific expertise, we filter out these impractical suggestions before valuable resources are wasted.
Generative chemistry complements other computational approaches
While generative chemistry identifies new molecule ideas, traditional computational approaches like QSAR (quantitative structure-activity relationship) models, and 3D docking and ligand-based methods, excel at evaluating and ranking existing compound libraries. These methods can also help us identify the most promising candidates from the many ideas proposed by generative chemistry methods.
ADMET (adsorption, distribution, metabolism, excretion and toxicity) prediction tools further enhance this workflow by assessing whether compounds are likely to have acceptable pharmacokinetics and safety profiles.
When chemists use all these tools together, we can create a powerful feedback loop. By generating, evaluating and optimizing in a continuous cycle, we can better identify the optimal molecules that are most likely to succeed for a given discovery project, and dramatically reduce the time and resources wasted synthesizing unsuccessful compounds.
What real-world impact can we expect in drug discovery?
Firstly, there's the advantage of speed. Generative chemistry can mine vast chemical space at a rate impossible for human teams alone. By rapidly evaluating new molecules in silico, we can compress months of iterative design work into days or even hours, allowing faster progression through hit-to-lead and lead optimization stages.
Secondly, it expands our exploration of chemical diversity. By generating structures that chemists might never have considered, these systems help overcome our natural tendency toward familiar chemical matter. This isn't just about being novel for novelty's sake – it's about finding truly differentiated compounds with unique properties.
Finally, combining generative chemistry with multi-parameter optimization leads to compounds with a higher chance of success. Drug candidates must simultaneously satisfy numerous criteria, from target potency to ADMET and physicochemical properties, such as metabolic stability, solubility and safety profiles (Fig 1). Advanced generative systems can navigate these complex, multi-dimensional constraints more systematically than traditional approaches, potentially reducing costly late-stage failures. 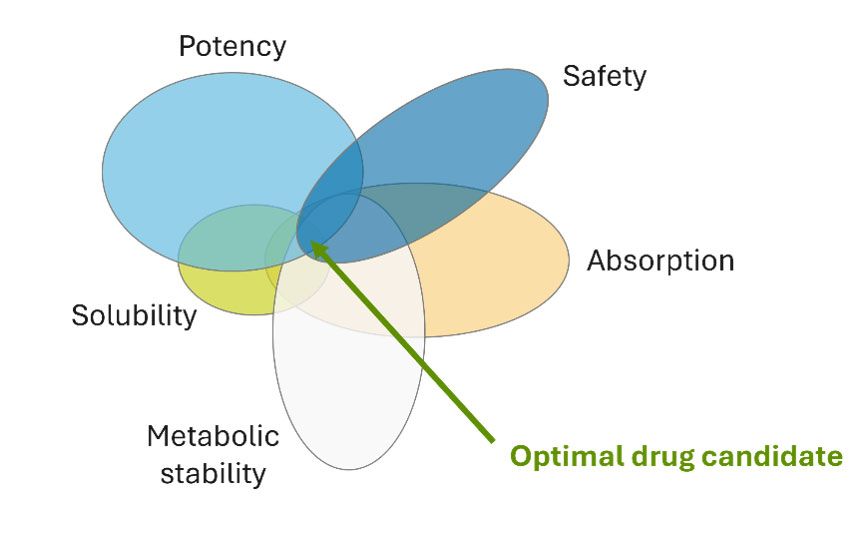
Figure 1: Multiparameter optimization in drug discovery: successful drug candidates must balance potency with other desirable ADMET and physicochemical properties. Credit: Optibrium.
Separating hype from reality
To separate hype from reality, we must maintain measured expectations. Generative chemistry won't instantly solve all drug discovery challenges or replace skilled scientists. What it offers is a powerful tool to accelerate discovery.
The most successful implementations carefully integrate these technologies into existing workflows, empowering scientists rather than attempting to automate their expertise away. When deployed thoughtfully, generative chemistry becomes a force multiplier for research teams, enabling them to identify promising compounds that might otherwise remain undiscovered.
In summary
As generative chemistry continues to evolve, the boundary between human and machine contributions will increasingly blur. However, even in the most autonomous systems, expert chemists should still make the decisions that require context and combinations of different types of information to achieve optimal results.3
AI won’t replace chemists, but chemists who leverage AI to their advantage will replace those who don’t. The most effective drug discovery programs will be those that embrace this complementary approach, combining the experience and intuition of scientists with the systematic exploration capabilities of machine learning.
In this way, we can navigate the vast chemical universe more efficiently than ever before, ultimately accelerating the discovery of life-changing treatments for patients worldwide.
References
1. Reymond JL. The chemical space project. Acc Chem Res. 2015;48(3):722-730. doi: 10.1021/ar500432k
2. Alakhdar A, Poczos B, Washburn N. Diffusion models in de novo drug design. J Chem Inf Model. 2024;64(19):7238-7256. doi: 10.1021/acs.jcim.4c01107
3. Goldman B, Kearnes S, Kramer T, Riley P, Walters WP. Defining levels of automated chemical design. J Med Chem. 2022;65(10):7073-7087. doi: 10.1021/acs.jmedchem.2c00334